How To Find Heat Released

Heat of Solution or Enthalpy of Solution Chemistry Tutorial
Key Concepts
⚛ A solute dissolves in backlog solvent to form a solution:
solute + solvent → solution
⚛ Rut of solution, or, enthalpy of solution, is the energy released or absorbed when the solute dissolves in the solvent.
⚛ Molar heat of solution, or, molar enthalpy of solution, is the energy released or captivated per mole of solute beingness dissolved in solvent.
⚛ Heat of solution (enthalpy of solution) has the symbol(one) ΔHsoln
⚛ Molar oestrus of solution (molar enthalpy of solution) has the units(two) J mol-1 or kJ mol-one
⚛ If heat is released when the solute dissolves, temperature of solution increases, reaction is exothermic, and ΔH is negative (ΔH < 0).
solute + solvent → solution ΔHsoln = −
solute + solvent → solution ΔHsoln < 0
⚛ If estrus is captivated when the solute dissolves, temperature of solution decreases, reaction is endothermic, and ΔH is positive (ΔH > 0).
solute + solvent → solution ΔHsoln = +
solute + solvent → solution ΔHsoln > 0
⚛ Enthalpy (heat) of solution can exist determined in the laboratory by measuring the temperature change of the solvent when solute is added.
⚛ To calculate the molar enthalpy of solution (molar heat of solution) using experimental information:
Step 1: Summate the amount of energy released or captivated (q)
q = m × Cgrand × ΔT
q = amount of energy released or absorbed
m = mass
Cg = specific heat capacity
ΔT = modify in temperature
Step 2: Calculate moles of solute (n)
due north = m ÷ M
northward = moles of solute
m = mass of solute
M = molar mass of solute
Pace three: Summate mount of free energy (heat) released or captivated per mole of solute (ΔHsoln)
ΔHsoln = q ÷ n
ΔHsoln = molar enthalpy (rut) of solution
q = amount of energy (heat) released or absorbed
n = moles of solute
Note: You must include the sign for ΔHsoln (either + or −)
(i) ΔH is negative if energy (oestrus) is released (exothermic).
(two) ΔH is positive if energy (heat) is captivated (endothermic).
Please practise non cake ads on this website.
No ads = no money for us = no complimentary stuff for you!
Theory Behind Determining Molar Enthalpy of Solution
The molecules or ions making upwards a solid solute exist in a highly ordered state which is referred to as a lattice.
You are probably already familiar with representations of ionic compounds in which positive ions (cations) and negative ions (anions) are bundled in a lattice held together by electrostatic forces of attraction known as ionic bonds.
The amount of disorder, or randomness, in a system is known as its entropy. A lattice is highly ordered, that is, the particles making up the lattice are in a depression state of disorder. This is referred to every bit a low entropy state(iii).
When this solute dissolves in a solvent, particles such as ions must be removed from the lattice and each solute particle must and then exist completely surrounded by solvent molecules.
The solute particles in the solution are in abiding motion and distributed more or less randomly throughout the solution so that the amount of disorder has increased compared to when they were part of the lattice. Solute particles in the solution are said to be in a college state of entropy than solute particles making up the lattice.
The process of dissolving a solid solute in a liquid solvent tin can therefore exist idea of every bit occurring in two steps:
If Enthalpy of Solution (estrus of solution) is Endothermic:
The magnitude of the free energy captivated to suspension up the lattice, ΔHlat, is greater than the magnitude of the energy released when solute particles are surrounded past water solvent molecules, ΔHhyd, so the enthalpy of solution, ΔHsoln, is positive, that is, the process is endothermic.
if |ΔHlat| > |ΔHhyd| and so ΔHsoln is positive
| solute(southward) + water(fifty) → solution(aq) ΔHsoln = + solute(southward) + h2o(l) + ΔHsoln → solution(aq) Energy is captivated, that is, energy is a reactant. When solute is added to water, h2o temperature decreases. |
|
If Enthalpy of Solution (estrus of solution) is Exothermic:
The magnitude of the energy absorbed to break upward the lattice is less than magnitude of the energy released when solute particles are surrounded by h2o solvent molecules , then the enthalpy of solution is negative, that is, the process is exothermic.
if |ΔHlat| < |ΔHhyd| and then ΔHsoln is negative
| solute(s) + water(l) → solution(aq) ΔHsoln = - solute(south) + h2o(l) → solution(aq) + ΔHsoln Free energy is released, that is, energy is a product. When solute is added to water, water temperature increases. |
|
The tabular array below lists the values of molar enthalpy of solution (molar heat of solution) for some mutual salts in water at 25°C.(4)
| Tooth Enthalpy of Solution of Some Salts at 25°C | |||||
|---|---|---|---|---|---|
| Endothermic examples | Exothermic examples | ||||
| Anion | solute | ΔHo soln (kJ mol-1) | solute | ΔHo soln (kJ mol-1) | |
| chloride | NHfourCl(due south) | +xiv.78 | LiCl(s) | -37.03 | |
| NaCl(s) | +3.88 | ||||
| KCl(s) | +17.22 | ||||
| bromide | NHivBr(s) | +xvi.78 | LiBr(s) | -23.26 | |
| KBr(due south) | +xix.87 | NaBr(south) | -0.60 | ||
| nitrate | NHivNOiii (s) | +25.69 | LiNO3 (south) | -2.51 | |
| AgNOiii (s) | +22.59 | ||||
| NaNOthree (s) | +twenty.50 | ||||
| KNOiii (s) | +34.89 | ||||
| hydroxide | LiOH(south) | -23.56 | |||
| NaOH(due south) | -44.51 | ||||
| KOH(s) | -57.61 | ||||
| acetate | NH4C2HiiiOtwo (s) | -2.38 | |||
| NaC2HthreeO2 (s) | -17.32 | ||||
| KC2HiiiO2 (s) | -15.33 | ||||
↪ Back to top
Experiment to Determine the Tooth Oestrus of Solution (Molar Enthalpy of Solution) of a Solute
The following describes the use of a polystyrene foam (styrofoam™) cup every bit a calorimeter to determine the heat of solution of a salt (solid solute) in h2o (the solvent).(5)
Polystyrene cream (styrofoam™) is a good insulator, that is, it is a material that does not behave rut well.
In this experiment the polystyrene foam (styrofoam™) cup is used as an insulated vessel in which to conduct the experiment and then that either:
(i) all the energy released by the reaction is used to raise the temperature of the water in the cup and is not lost in heating the surrounding environment in the case of an exothermic reaction
(ii) the but energy being captivated past the reaction is that from the h2o in the cup and not from the surrounding surround in the case of an endothermic reaction.
Procedure:
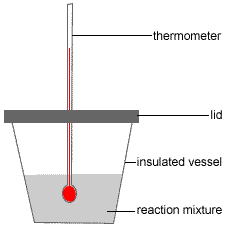
- Step one: An accurately known quantity of water (the solvent) is placed in a well insulated vessel (eg, a polystyrene foam or styrofoam™ cup)
- Pace two: The initial temperature of this solvent is recorded, Ti.
- Step three: An accurately known quantity of the solid solute is added, the vessel is sealed with a chapeau and the solution stirred using the thermometer.
- Step 4: The final temperature of the solution is recorded, Tf.
Note the final temperature will be the maximum temperature reached for an exothermic reaction or the minimum temperature reached for an endothermic reaction.
↪ Dorsum to top
Calculation of Molar Enthalpy of Solution (tooth rut of solution) (6)
Step 1: Calculate the estrus released or absorbed, in joules, when the solute dissolves in the solvent:
heat released or absorbed = mass × specific heat capacity × change in temperature
q = m × cthou × (Tconcluding - Tinitial )
q = m × cg × ΔT
| What is the value of mass, m? In that location are 2 ways to expect at this: | ||
| 1. Method usually used by High School courses. Presume all the solute dissolves simultaneously in the solvent and so that all the heat is simultaneously captivated from, or released to, the pure solvent. k = mass of solvent in grams So, c1000 = specific heat capacity of the solvent in J°C-1g-1 ΔT = Tfinal - Tinitial in °C(7) When calculated, q is in joules (J) | OR | two. Method usually used in introductory University courses. Presume the showtime molecule or ion of solute dissolves into pure solvent, simply each subsequent molecule or ion is dissolving into a mixture of dissolved solute in solvent (that is, a solution). In this case, the terminal solute molecule or ion is dissolving into a solution with a mass approximated past the mass of the solvent plus the mass of the solute. k = mass of solvent + mass of solute in grams So, cg = specific heat capacity of the solution in J°C-1m-i ΔT = Tfinal - Tinitial in °C(seven) When calculated, q is in joules (J) |
Step 2: Calculate moles of solute:
moles = mass ÷ molar mass
where:
moles = amount of solute in mole
mass = mass of solute in grams
molar mass = molar mass of solute in grams per mole
Stride 3: Calculate the molar enthalpy of solution, or molar heat of solution, ΔHsoln:
| ΔHsoln in J per mole of a solute is calculated: | ΔHsoln in kJ per mole of a solute is calculated: |
|---|---|
| ΔHsoln = q ÷ moles of solute (J mol-i) where exothermic reactions: ΔHsoln is negative endothermic reactions: ΔHsoln is positive | ΔHsoln = q/1000 ÷ moles of solute (kJ mol-1) where exothermic reactions: ΔHsoln is negative endothermic reactions: ΔHsoln is positive |
↪ Dorsum to pinnacle
Worked Example: Sample Experimental Results and Calculations
Question:
A educatee added iv.00 1000 of NaOH(southward) to 100 g of h2o in a polystyrene foam loving cup.
The temperature of the water rose by x.0°C.
Assuming the polystyrene foam cup is well insulated and the specific heat capacity of h2o is 4.18 J°C-1g-ane, determine the molar enthalpy of solution of sodium hydroxide in kJ mol-1.
Solving the Problem using the StoPGoPS model for problem solving:
| STOP | STOP! State the Question. | |||||||||||||||||||||||||||||||||
| What is the question asking you to do? Calculate the molar heat of solution of sodium hydroxide in kJ mol-1 ΔHsoln = ? kJ mol-i | ||||||||||||||||||||||||||||||||||
| Break | Pause to Set a Game Plan | |||||||||||||||||||||||||||||||||
| (1) What information (data) have you been given in the question? m(NaOH) = mass NaOH(s) = 4.00 g m(H2O) = mass HiiO(l) = 100 g ΔT = temperature increase = 10.0°C cg(H2O) = specific oestrus capacity of water = 4.18 J°C-1yard-i (2) What is the relationship between what you know and what you need to discover out?
Note: ΔHsoln will exist negative considering the temperature increased, that is, the procedure is exothermic. | ||||||||||||||||||||||||||||||||||
| GO | Go with the Game Program | |||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||
| PAUSE | Suspension to Ponder Plausibility | |||||||||||||||||||||||||||||||||
| Is your answer plausible? Work backwards:
Since this value for the change in temperature is the aforementioned as that given in the question, nosotros are confident our respond is correct. | ||||||||||||||||||||||||||||||||||
| STOP | STOP! State the Solution | |||||||||||||||||||||||||||||||||
| State your solution to the trouble. Method 1: Molar enthalpy of solution of sodium hydroxide is −41.8 kJ mol-1 Method 2: Tooth enthalpy of solution of sodium hydroxide is −43.5 kJ mol-one | ||||||||||||||||||||||||||||||||||
↪ Back to top
Sample Question
A student added 1.92 g of KBr(southward) to 100 g of water in an insulated vessel. The temperature of the h2o fell by 0.766°C. Calculate ΔHsoln in kJ mol-i.
↪ Back to top
Footnotes:
(1) If the solute and the solvent are in their standard states, you tin can too write ΔHo sol
Refer to Standard Enthalpy (oestrus) of Germination and Reaction
(2) You may also units of cal mol-ane or kcal mol-1
i calorie = 4.18 joules
1 cal = 4.18 J
For conversions between J, kJ, and cal refer to energy conversions
(3) Entropy refers to the amount of disorder, or randomness, in a organisation.
Ions locked into a crystal structure are in a low state of disorder and therefore accept low entropy.
Ions moving randomly through the solvent molecules in a solution are much more than matted, much more than random, and then they are said to be in a higher country of entropy.
Gas molecules moving randomly in the atmosphere are in an even greater country of disorder or randomness, and then they are said to accept an even higher country of entropy.
Moving towards a higher state of entropy is one of the driving forces for a chemical reaction.
(4) The values given are for solutions at space dilution which is the enthalpy change when 1 mole of solute in its standard state is dissolved in an infinite amount of h2o.
(5) Go to Calorimetry Tutorial to see an alternative method used an adiabatic solution calorimeter.
(6) On this page the calculations assume that at that place is a large excess of solvent
Note that the post-obit examples all employ the specific rut chapters of the solvent, c1000, not molar heat capacities, C.
(7) You lot can employ other units for temperature.
If you use Kelvin (K), and then cm will have the same numbers but the units change to JK-1thousand-1. This because a change of temperature of 1K is the same every bit the change of temperature of 1°C.
If yous use fahrenheit and then you will have to apply a value for cg that has the units J°F-1m-1
↪ Back to top
Source: https://www.ausetute.com.au/heatsolution.html
Posted by: hayesfacithe.blogspot.com


0 Response to "How To Find Heat Released"
Post a Comment